Navigation
Understanding Neural Crest Development at the Intracellular Level
The neural crest is a transient population of multipotent cells in the vertebrate embryo. Its development spans gastrulation, neurulation, and organogenesis. It can be divided into five steps: neural border induction, neural crest induction, epithelium-to-mesenchyme transition (EMT), migration, and final differentiation into diverse tissues including the sympathoadrenal lineage.
It is known that amplification of the MYCN oncogene and activating mutations of the ALK oncogene can transform neural crest progenitor cells into neuroblastoma cells, which can interconvert between two phenotypes, N-type (adrenergic) and S-type (mesenchymal) cells, which have different levels of chemoresistance and invasive properties.
While working on the PRIMAGE project, I became interested in identifying the exact developmental step where this transformation occurs and explaining it in terms of intracellular molecular details. I conceived this project and secured an Insigneo research grant to fund it.
First Approach
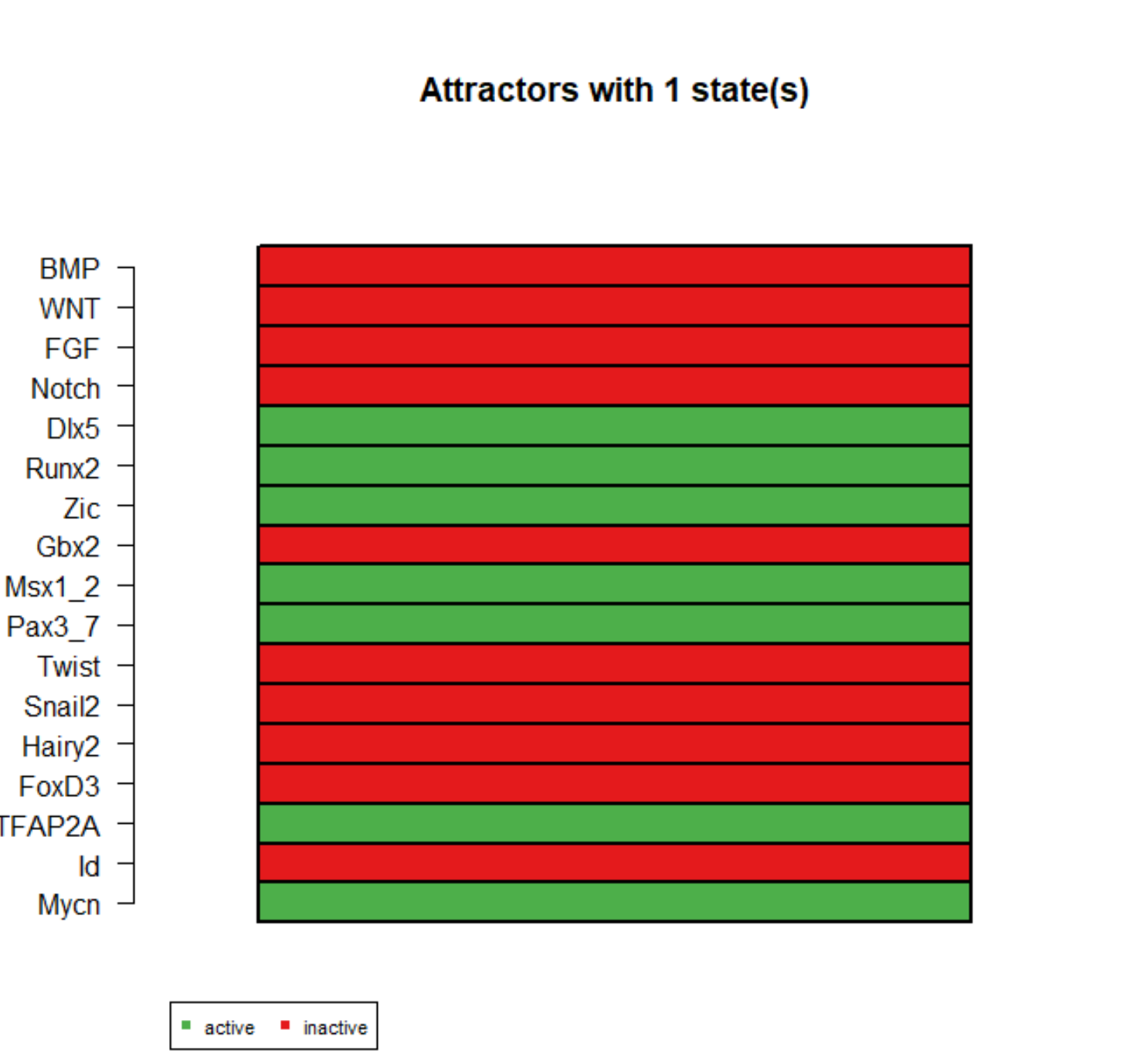
In the academic year 2019–2020, Jack Ashurst carried out his undergraduate thesis research (computer science) at the University of Sheffield. Doctor Dawn Walker and I co-supervised him. He modelled the first two steps with a top-down approach. Within a minimalist Boolean network, he connected the BMP, Wnt, FGF, and Notch signalling pathways to the neural border and neural crest specifiers, which constitute a gene regulatory network. He searched for attractors under a synchronous updating scheme and an asynchronous one. He reproduced two experiments: the model's inductive signals upregulated the specifiers therein and a knock-down experiment.
The neural border specifiers were known to switch off after the second step, so he perturbed an attractor by withdrawing the inductive signals and overexpressing MYCN in the model. Despite an upregulated MYCN, the neural border specifiers failed to stay active in the model. It is possible that MYCN amplification does not impair the first two steps. However, a more plausible reason is that some regulatory mechanisms are elusive. Related is the fact that multiple events occur in each step; their regulatory mechanisms overlap but differ.
A Different Perspective
After considering Jack's results, I decided to switch to a bottom-up approach and focus on a well-characterised fundamental process carried out by neural crest progenitor cells: asymmetric division.
In 2022, Alvaro Andre Yupanqui Rivera, an undergraduate bioengineer, joined the project as an Insigneo summer intern. Within a probabilistic Boolean network, we modelled the intracellular mechanism regulating this process. Using Tarjan's strongly connected components algorithm, we searched for ergodic sets with different input nodes knocked out. Each of them corresponds to a phenotype favouring asymmetric division or symmetric division.
At this point, I decided to focus on advancing therapy for neuroblastoma rather than studying its origin, so I ended the project.