Navigation
Predictive in Silico Multiscale Analytics to Support Cancer Personalised Diagnosis and Prognosis, Empowered by Imaging Biomarkers (PRIMAGE)
Between February 2019 and December 2022, I was a part of this 10 million–euro Horizon 2020 project. It was launched by 16 institutions from eight European countries to build a cloud-based decision-making platform for the clinical management of malignant solid tumours, specifically predictive tools for diagnosis, prognosis, therapeutic choices, and treatment follow-up, including novel imaging biomarkers, in silico tumour growth simulation methods, advanced visualisation techniques, and machine learning tools. This video explains the PRIMAGE project's relevance to the clinical management of paediatric cancers: link.
Based at the University of Sheffield, I modelled two paediatric cancers at the cellular scale under the supervision of Doctor Dawn Walker.
Neuroblastoma
I built a computational model of neuroblastoma by combining a continuous automaton, autonomous agents, and a centre-based mechanical model.
The continuous automaton, which is a generalised cellular automaton, divides the tumour into voxels. Each voxel is associated with an amount of extracellular matrix, a neuroblastoma cell count, and a Schwann cell count. The grid is itself associated with a homogeneous amount of oxygen and nutrients, as well as an extent of inflammation. The major agent behaviours are cell cycling and cell death. These are regulated by the microenvironment and each agent's internal attributes. For example, hypoxia can damage an agent's DNA and thus induce apoptosis, but the agent can repair its DNA if p53 is active. Some of the regulatory mechanisms are double-edged. For example, an apoptotic or necrotic agent is easier to remove in a more inflamed microenvironment, but inflammation also leads to further necrosis. The two agent populations interact via the continuous automaton, which records their spatial distributions. While neuroblastoma cells promote cell cycle progression in Schwann cells, Schwann cells induce apoptosis and differentiation in neuroblastoma cells; a more differentiated neuroblastoma cell is less proliferative. When two agents overlap, contact inhibition stops cell cycling and matrix production. The centre-based mechanical model resolves the conflict.
Then, I aggregated data about neuroblastoma's histology, mutations, hypoxia, and growth kinetics. Together, the tumour's histology type and mutation profile determine the split between the two agent populations, the differentiation state of the neuroblastoma cell population, their ability to repair telomeres, and their cell cycling rate. I developed a tournament-style pipeline based on Latin hypercube sampling to calibrate the model on the basis of the aggregated data.
Using the calibrated model, I simulated the dynamics of clonal competition within heterogeneous tumours and evaluated combinations of targeted therapies on the GPUs on the Bessemer high-performance computing cluster. I analysed the resulting datasets by dimensionality reduction, clustering, and hypothesis testing. I theorised about an unconventional therapy based on targeting p53's regulators. Although p53 is usually considered a tumour suppressor gene, the protein it encodes repairs damaged DNA too. My theory is that p53 is on balance pro-apoptosis in most neuroblastoma cells, but a typical tumour contains a small portion of cells wherein it is pro-survival. It follows from my theory that the usual strategy of activating p53 and stabilising p53 is incomplete. An adaptive approach is worth exploring.
To accelerate the costly simulations, I built surrogate models by multiple linear regression and deep learning.
In parallel, I collaborated with the University of Zaragoza, University of Bologna, and Chemotargets SL to integrate the calibrated model with their organ-scale and single-cell models with a new scale separation method.
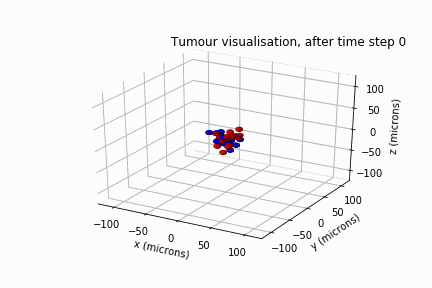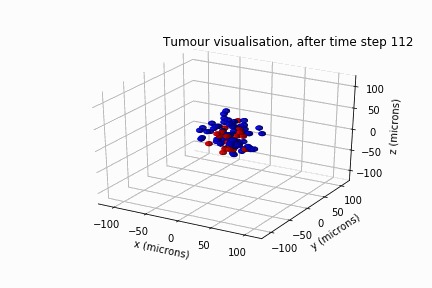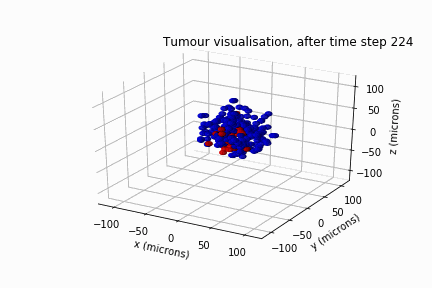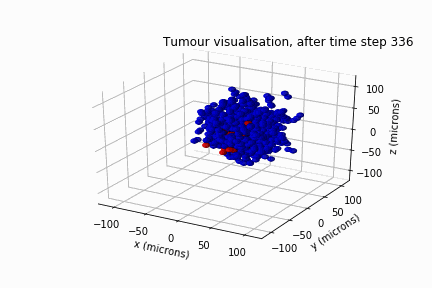
Diffuse Intrinsic Pontine Glioma
In June 2021, Will Shaw, a computer science undergraduate student at our university, joined Dr Walker and me for a summer project funded by the SURE (Sheffield Undergraduate Research Experiences) scheme. He spent the summer reviewing the literature on diffuse intrinsic pontine glioma (DIPG) thoroughly.
In May 2022, Luke Jones, another undergraduate student from the same department, and I secured a bursary from the Sheffield Undergraduate Research Experience (SURE) scheme. Based on the results of Will's review, we modelled DIPG's clonal evolution and invasive behaviour in a biological lattice-gas cellular automaton.